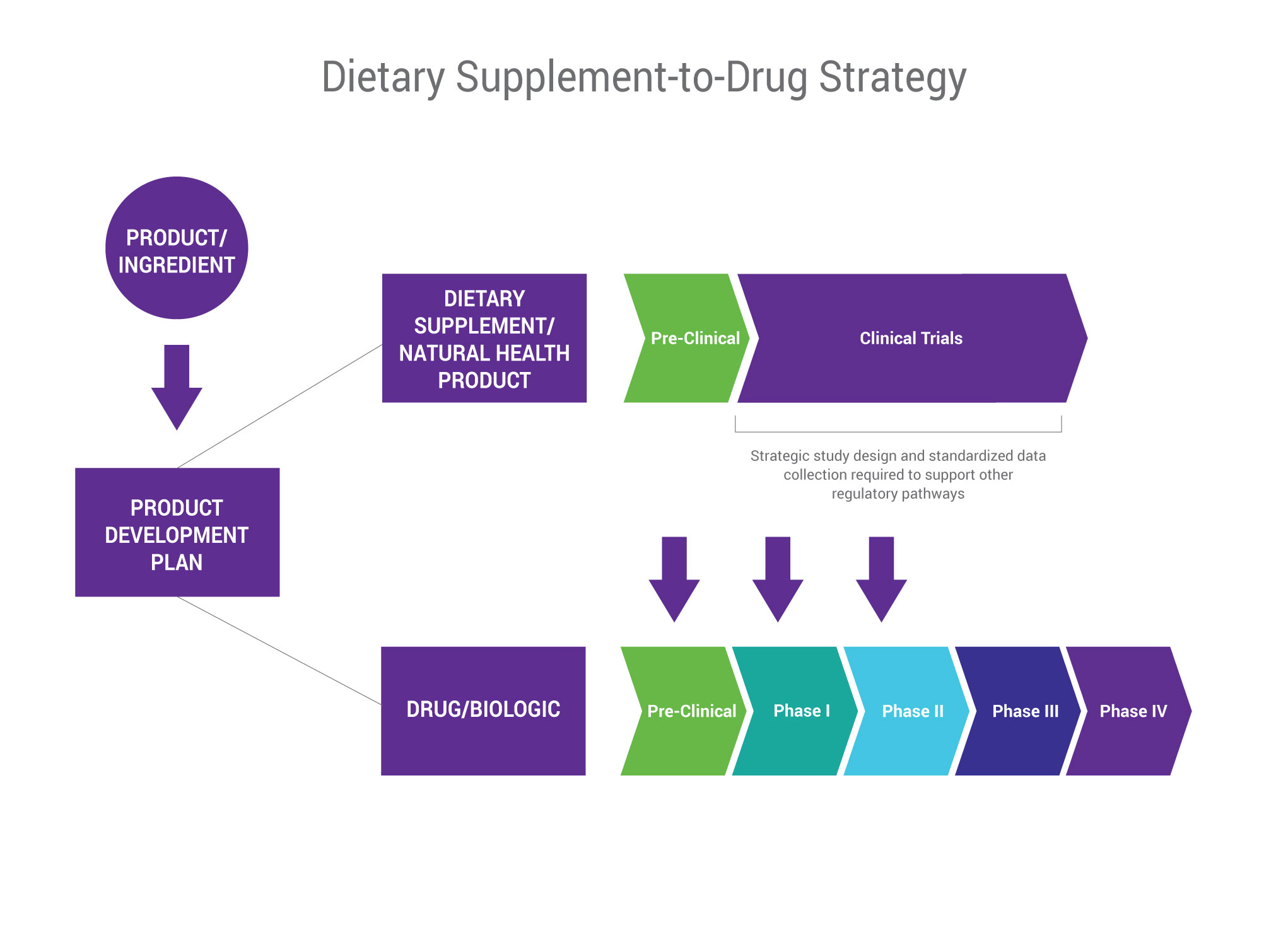
The opportunity to develop a dietary supplement into a pharmaceutical has never been greater. As drug pipelines run dry of new molecular entities, and supplement claims become increasingly aggressive, the two categories are converging in a way that presents limitless market possibilities.
With this potential comes the need for an experienced CRO that understands the regulatory complexities of both supplement and pharmaceutical categories while thinking multiple steps ahead. At SGS Nutrasource, we pride ourselves on fitting into this niche. With 15+ years and a successful track record in dietary supplement and pharmaceutical regulatory and clinical consulting, SGS Nutrasource provides the expertise you need to strategically and confidently transition your supplement into a drug.
Our specialized product development approach, outcome measurement determination, and protocol generation can assist with the planning and regulatory/clinical requirements needed to develop ingredients from nutraceutical to pharmaceutical with realistic goals, budgets, and timelines.
Our clients benefit from:
- A specialized clinical development approach tailored to each client’s needs and goals
- Full, in-house project management teams, each with a technical Project Manager (10+ years of experience) to oversee your project
- Teams with 120+ combined years in global regulatory strategy for both pharmaceuticals and dietary supplements/natural health products (NHPs)
- Long-term working relationships with government authorities including the U.S. FDA and Health Canada
- Successful track record in drug scale-up and development, including defining the product label, filing patents, conducting early clinical development /pre-clinical and clinical studies (Phase I, Phase II/III , and Phase IV), and submitting Investigational New Drug submissions (INDs) and New Drug Applications (NDAs)
Learn how we can help you unlock the pharmaceutical potential of your dietary supplement.
 Skip to main content
Skip to main content